Medical beds can also be called medical beds, medical beds, nursing beds, etc. They are beds used by patients when they are hospitalized. They are mainly used in major hospitals, township health centers, community health service centers, etc.
The US FDA requires that when food and medical products enter the US market, they must be registered on the US FDA official website before they can enter the US market.
Hospital beds are classified as Class I medical devices in the FDA. The U.S. Food and Drug Administration defines Class I devices as “not intended to be used to sustain life or sustain life, or to be important in preventing harm to human health, and may not present potential “Devices that pose an unreasonable risk of illness or injury.” These devices are the most common category of devices regulated by the FDA, accounting for 47% of approved devices on the market. Class I devices have minimal patient contact and have minimal impact on the patient’s overall health. Typically, Class I devices do not come into contact with the patient’s internal organs, central nervous system, or cardiovascular system. These devices are subject to minimal regulatory requirements.
FDA certification of medical devices includes: manufacturer registration with FDA, product FDA registration, product listing registration (510 form registration), product listing (PMA review), labeling and technical transformation, customs clearance, registration, and pre-market reporting of medical and health care devices, The following materials must be submitted:
(1) Five copies of fully packaged finished products
(2) Device structure diagram and text description
(3) Performance and working principle of the device
(4) Safety demonstration or test materials of the device
(5) Introduction to manufacturing process
(6) Summary of clinical trials
(7) Product instructions. If the device has radioactive properties or releases radioactive materials, it must be described in detail.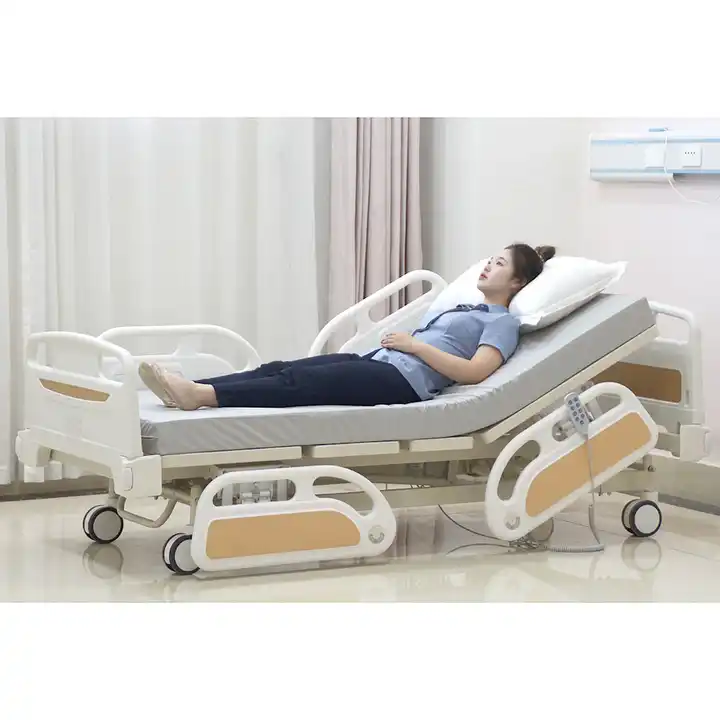
Project Cycle
The time from FDA’s evaluation to final approval is generally longer and is controlled by the FDA; usually the entire normal process cycle is about 12 months
The 510K application process for hospital beds is as follows:
1. FDA 510(K) technical document compliance requirements
2. Standard analysis applicable to US FDA 510k registration
3. Confirmation of availability of existing documents
4. Collection and comparison of registered products on the market
5. Prepare product information in accordance with US FDA 510k requirements
6. Prepare 510k registration documents according to standards
7. Make revisions based on the review results of the registration documents
8. Complete company registration and product listing registration
taishaninc has global export certification
It has 5 wholly-owned subsidiaries
Covering building materials, chemicals, and medical device industries
We are a factory with global export certification, with an annual output value of $5,000,000 and exports to more than 160 countries around the world. We are the largest integrated industrial park factory in the local area. If necessary, please contact us in time and send detailed product information.
Post time: Nov-21-2023